
The fission diagram below shows the uncontrolled chain reaction that would occur from the fission of uranium-236, leading to the production of barium-144 and krypton-89. This is the principle behind nuclear fission reactors, which use uranium as a fuel source to produce controlled amounts of energy. However, if the number of product neutrons used to initiate further fissions can be controlled, then the total amount of energy released can also be controlled. This principle was used to build atomic bombs, which can release an enormous amount of energy and destroy entire cities. In an uncontrolled chain reaction, the number of fissions grows exponentially with time, leading to a huge release of energy in a short amount of time. These neutrons can be used to cause further nuclear fissions, leading to a chain reaction that can release even more energy. Nuclear fission, which involves the splitting of heavy nuclei into smaller nuclei, can lead to the production of moving neutrons and an enormous amount of energy. The fission products (barium, krypton and nuclei) all have some kinetic energy after the fission occurs. The barium and krypton nuclei may undergo alpha and beta decay and form even smaller nuclei. Two neutrons are released in the process and of energy (this is equivalent to). It then splits into barium-139 and krypton-95 which are both smaller nuclei. Two neutrons are released in the process along with 200 MeV of energyĪ neutron is fired into a stable uranium-235 nucleus, making it momentarily unstable. When a neutron collides with the nucleus of uranium-235, the nucleus splits into barium-139 and krypton-95, releasing two or three neutrons, gamma rays, and kinetic energy in the process. Part of the energy that is released is in the form of the kinetic energy of the fission products.Īn example of this process is the fission of uranium-235 into barium-139 and krypton-95. The smaller nuclei, known as the fission products, are usually also unstable and may release alpha or beta particles to attain stability.

The nucleus then splits into two smaller nuclei, that are similar in size, and releases two or three neutrons in the process along with large amounts of energy in the form of gamma rays. After the neutron collides with the nucleus, it causes the nucleus to become unstable.
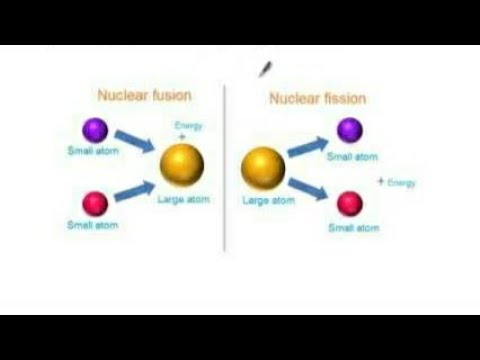
It is not a random process, but requires a neutron to collide with the heavy nucleus for the splitting to occur.

Nuclear fission is the process of splitting a heavy atom into two or more smaller nuclei, releasing a large amount of energy in the process. On the other hand, heavier nuclei can be broken apart into smaller ones, which generates some energy. This is called nuclear fusion, and it releases energy. When the nuclei of smaller atoms collide and merge, they can sometimes form heavier atoms. In this article, we'll explore fission and fusion, which are the splitting and combining of nuclei. Scientists have discovered that we can split or fuse nuclei, and now we're eager to understand the energies involved in these reactions. These tiny components are responsible for some of the most energetic reactions on Earth, which can be both constructive and destructive. The heavier the atom, the more protons and neutrons it has in its nucleus. The nucleus is made up of neutrons and protons, which are held together by a strong nuclear force. Every atom has a nucleus at its core that makes up more than 99% of its mass.


 0 kommentar(er)
0 kommentar(er)
